Making Recombinant Antibodies in Plants
In this blog we will look at how we make recombinant antibodies in plants, and importantly, why?
Purified antibodies are a powerful tool used throughout the life sciences sector; be that for clinical use to deliver therapeutic action against diseases such as cancer, diagnostics to confirm a person’s response to infection or allergens, or to support fundamental experiments within research labs. With such extended use, being able to produce purified antibodies cheaply and quickly provides huge benefits to the industry and the wider community. With all this in mind, how do we make recombinant antibodies in plants and why would we want to?
Producing antibodies in plants – How it works
Leaf Expression Systems (Leaf) technology uses a plant called Nicotiana benthamiana, which is a plant native to Australia. The plant is widely used within plant virology research as an experimental organism due to a large number of plant pathogens able to infect it.
The expression of monoclonal antibodies in plants is simple when using Leaf’s system – the antibody’s DNA sequence, made up of the ‘heavy and light chains’ are placed into the system’s vector, either individually or together and introduced into Argobacterium tumefaciens (plant pathogen) by the process of transformation. This bacterium is then used to pass the vector into the plants’ leaf cells through a process called infiltration.

The image above demonstrates a small-scale infiltration, larger-scale infiltration is performed in tanks held under a vacuum. The plants are submerged in agrobacterium culture fluid and upon release of the vacuum the bacteria are taken up through pores and into the spaces between the plant cells, whereby it introduces Leaf’s vectors into the plant cell. This process creates plants that contain copies of the vector which carry the instructions needed to express the antibody.
Antibody expression is transient, meaning it is non-permanent and cannot be passed onto any future plant generations (the plants are not GMOs). Inserting the DNA instructions in this way, effectively turns the plants into individual bioreactors.
How do plants express antibodies?
The infiltrated plants start expressing the human antibody within their cells very quickly. As plants have no natural use for antibodies, the expressed material starts accumulating within the leaves’ cells. As an antibody is formed of two protein sequences, (heavy and light chains), the expression must happen in the same cell. The two chains are ‘made for each other’ and naturally come together within the plant cell to create the full antibody complex. The combination of chains is especially easy if all the recombinant material has been targeted to a specific area of the plant cell, and we can do this easily by adding additional pieces of information to the vector that tells the plant cell where the translated protein strands should be sent for processing (folding up into the correct structure). Because plants are a eukaryotic organism, they are able to carry out important modifications on fully formed antibodies – like adding sugar molecules to the surface (glycosylation). There are some differences in the modifications made by plants to what would happen naturally in a human cell, however, but the resulting differences very rarely affect the functionality of the antibody. If this is an issue for the function of a certain antibody, then there are plants available that have been genetically engineered to be able to mimic exactly the modification patterns produced by the machinery of human cells.
How are monoclonal antibodies purified?
For use in medicine or diagnostics, an antibody must be ‘pure’, ensuring it is free from all other proteins and contaminants that may come from the organism it was made inside. The antibody produced is known as a monoclonal antibody. These types of antibodies are important in medicinal applications as there are no variations in the antibody structure meaning you can be confident that its efficacy and behavior will always be the same.
Infiltrated plants are left to grow for up to 10 days and then the leaves are harvested ready for the purification process. To produce a pure monoclonal antibody it first needs to be efficiently extracted from the leaf tissue. Once extracted, the ‘smoothy-like’ sample is filtered and processed using affinity chromatography.
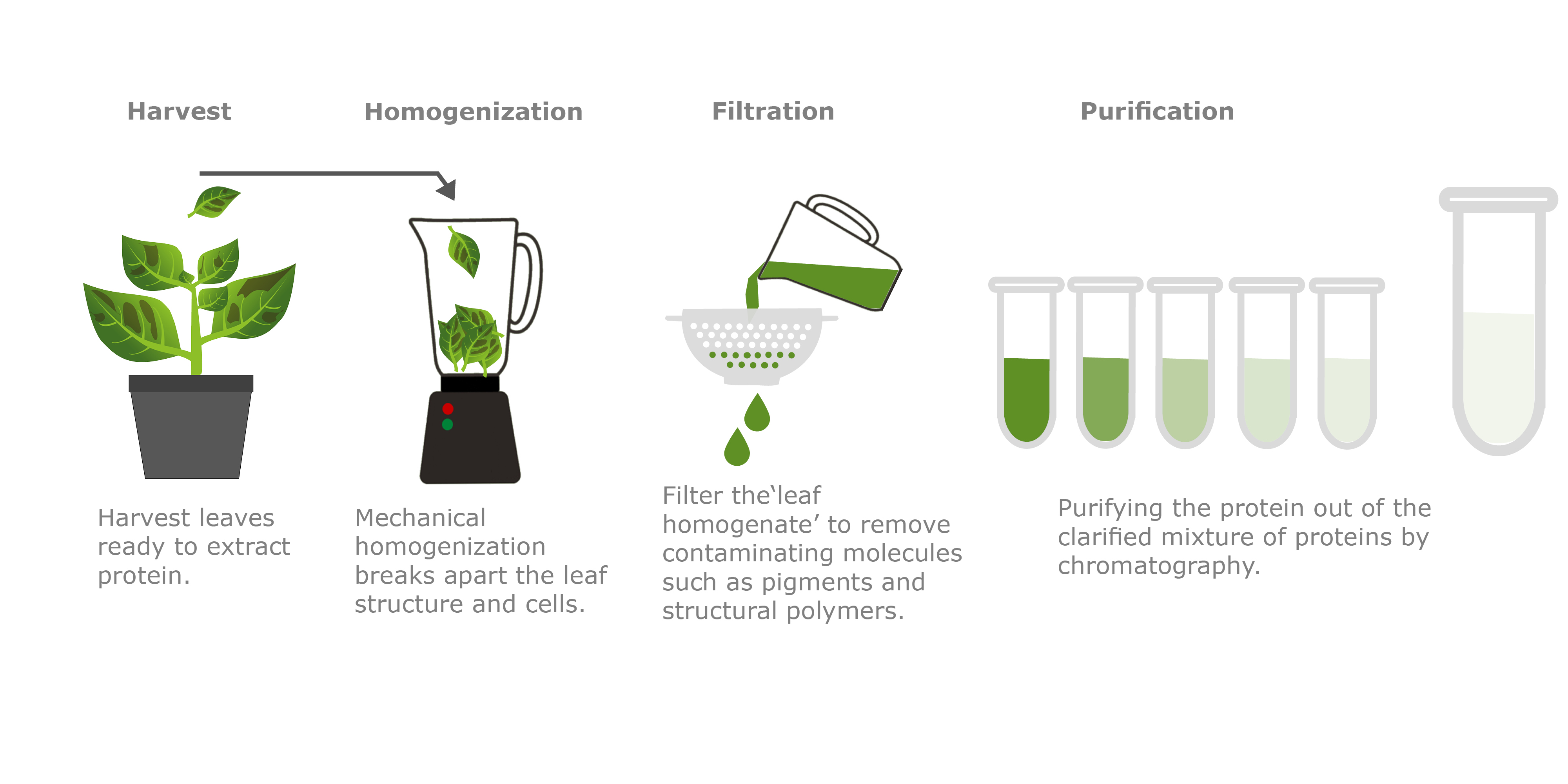
Affinity chromatography is a well-known method used in the purification of antibodies. This common technique relies on the interaction of Protein A with a specific region of the antibody (Fc region of IgG type antibodies). Protein A originates from the bacteria Staphylococcus aureus, where it is found on the surface of cell walls and has an affinity for immunoglobulin-like proteins, specifically antibodies of the IgG class. In nature Protein A can trick the immune system by binding to the receptor end of an antibody, preventing attack by the immune system. This binding affinity to a well-conserved antibody region is exploited in chromatography, where the column resin contains immobilized Protein A molecules. As the clarified sample from the purification process is applied to the chromatography resin, the immobilized Protein A molecules grab onto the antibody molecules and hold them on the column, whilst all the other proteins flow straight through. As the target antibody is the only antibody stuck to the Protein A resin, a pure preparation of the antibody can be obtained by “washing” the antibody off the column separately.
What are the benefits of using plants?
Related Blog: Top Reasons for Using Plants to Make Biologics
Plants offer a lot of positives in the production of pharmaceuticals. There is little crossover in terms of contamination for example; potentially dangerous contaminants such as mammalian viruses that must be controlled stringently in mammalian cell cultures do not occur in plants. Plants are also much simpler to produce and maintain than a hybridoma cell line as they simply need nutrients, water and light. Their simplicity enhances their scalability and decreases upstream costs; if you want more recombinant material, you just need to grow more plants.
At Leaf Expression Systems we believe plant-based expression to be a reliable and effective system to produce monoclonal antibodies. We see that there is great potential for plants to take their much-deserved place in the ever-expanding market of antibody production for therapeutic and diagnostic use against a multitude of human and animal diseases.
Written by Dr Claire A Fowler
